Cholinesterase antidotes
Faculty of Science UHK
Project Description
The cholinesterase reactivators are life savings antidotes applied in case of organophosphate poisoning. Our team is investigating novel entities for enhanced reactivation of organophosphate inhibited cholinesterases (acetylcholinesterase or butyrylcholinesterase). Up to now, we have developed numerous amount of novel reactivators (so-called oximes) that are able to efficiently reverse phosphylated cholinesterases in vitro or in vivo. Our compounds are worldwide well-known for excellent reactivation of organophosphorus pesticides and nerve agent tabun with excellent biocompatibility.
Projects
• 18-01734S – Butyrylcholinesterase reactivators for preparation of pseudo-catalytic scavengers applicable for organophosphorus intoxications
• 18-08937S – Research of oxime-CB(7) complexes for central nervous system penetration of quaternary acetylcholinesterase reactivator
• 8F17004 – Novel butyrylcholinesterase reactivators for pseudo-catalytic scavenging of organophosphates (BChER)
• TG02010020 – Development of novel nucleophiles for reactivation of cholinesterases inhibited by organophosphorus compounds
• LH13009 – The research of modified cholinesterase reactivators for organophosphorus pesticide poisoning treatment
Publications
• Antonijevic, E.; Kotur-Stevuljevic, J.; Musilek, K.; Kosvancova, A.; Kuca, K.*; Djukic-Cosic, D.; Spasojevic-Kalimanovska, V.; Antonijevic, B. Effect of six oximes on acutely anticholinesterase inhibitor-induced oxidative stress in rat plasma and brain. Archives of Toxicology, in press. DOI 10.1007/s00204-017-2101-z
• Kuca, K.*; Korabecny, J.; Dolezal, R.; Nepovimova, E.; Soukup, O.; Gorecki, L.; Musilek, K. TETROXIME: reactivation potency – in vitro and in silico study. RSC Advances. 2017, vol. 7, no. 12, p. 7041-7045. DOI: 10.1039/C6RA16499D
• Sahu, A.K.; Sharma, R.; Gupta, B.; Musilek, K.; Kuca, K.; Acharya, J.; Ghosh, K.K.* Oxime-mediated in vitro reactivation kinetic analysis of organophosphates-inhibited human and electric eel acetylcholinesterase. Toxicology Mechanisms and Methods. 2016, vol. 26, no. 5, p. 319-326. DOI: 10.3109/15376516.2016.1143070
• Nepovimova, E.; Korabecny, J.; Dolezal, R.; Nguyen, D.; Jun, D.; Soukup, O.; Pasdiorova, M.; Jost, P.; Muckova, L.; Malinak, D.; Gorecki, L.; Musilek, K.; Kuca, K.* 7-Methoxytacrine – 4-Pyridinealdoxime Hybrid as Novel Prophylactic Agent with Reactivation Properties in Organophosphate Intoxications. Toxicology Research, 2016, vol. 5, no. 4, p. 1012-1016. DOI: 10.1039/C6TX00130K
• Antonijevic, E.*; Musilek, K.; Kuca, K.; Djukic-Cosic, D.; Vucinic, S.; Antonijevic, B. Therapeutic and reactivating efficacy of oximes K027 and K203 against a direct acetylcholinesterase inhibitor. NeuroToxicology, 2016, vol. 55, no. 1, p. 33-39. DOI: 10.1016/j.neuro.2016.05.006
• Musilek, K.; Hambalek, J.; Holas, O.; Dohnal, V.; Kuca, K.* Monooxime bispyridinium reactivators bearing xylene linker; synthesis and in vitro evaluation on model of organophosphate-inhibited acetylcholinesterase. Medicinal Chemistry, 2016, vol. 12, no. 4, p. 362-370. DOI: 10.2174/1573406411666151002125640
• Kuca, K.*; Musilek, K.; Jun, D. TRISOXIME; a bulky trisquaternary reactivator of acetylcholinesterase. Letters in Drug Design & Discovery. 2016, vol. 13, no. 5, p. 372-375. DOI: 10.2174/1570180812666150923235240
• Winter, M.; Wille, T.; Musilek, K.; Kuca, K.; Thiermann, H.; Worek, F.* Investigation of the reactivation kinetics of a large series of bispyridinium oximes with organophosphate-inhibited human acetylcholinesterase. Toxicology Letters, 2016, vol. 244, no. 1, p. 136-142. DOI: 10.1016/j.toxlet.2015.07.007
• Musil, K.; Florianova, V.; Bucek, P.; Dohnal, V.; Kuca, K.; Musilek, K.* Development and validation of a FIA/UV-Vis method for pKa determination of oxime based acetylcholinesterase reactivators. Journal of Pharmaceutical and Biomedical Analysis, 2016, vol. 117, no. 1, p. 240-246. DOI: 10.1016/j.jpba.2015.09.010
• Zunec, S.*; Radic, B.; Kuca, K.; Musilek, K.; Lucic Vrdoljak, A. Comparative determination of the efficacy of bispyridinium oximes in paraoxon poisoning. Arhiv za Higijenu Rada i Toksikologiju, 2015, vol. 66, no. 2, p. 129-134. DOI: 10.1515/aiht-2015-66-2623
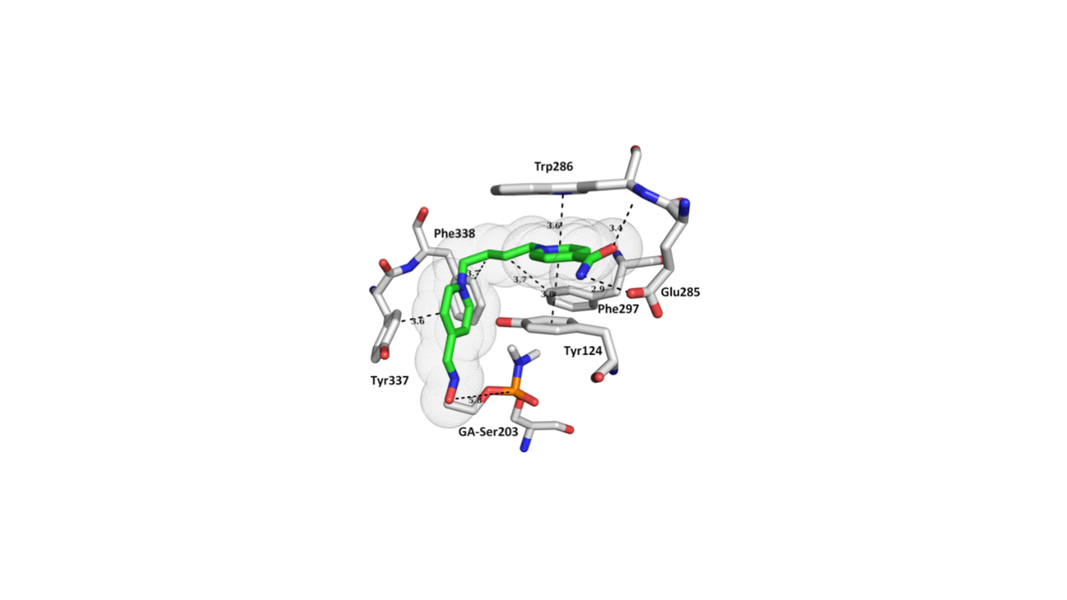
• Kovarik, Z.*; Macek Hrvat, N.; Katalinic, M.; Sit, R.; Paradyse, A.; Zunec, S.; Musilek, K.; Fokin, V.; Taylor, P.; Radic, Z.* Catalytic soman scavenging by Y337A/F338A acetylcholinesterase mutant assisted with novel site-directed aldoximes. Chemical Research in Toxicology, 2015, vol. 28, no. 5, p. 1036-1044. DOI: 10.1021/acs.chemrestox.5b00060
• Horn, G.; Wille, T.; Musilek, K.; Kuca, K.; Thiermann, H.; Worek, F.* Reactivation kinetics of 31 structurally different bispyridinium oximes with organophosphate-inhibited human butyrylcholinesterase. Archives of Toxicology. 2015, vol. 89, no. 3, p. 405–414. DOI: 10.1007/s00204-014-1288-5
• Dolezal, R.; Korabecny, J.; Malinak, D.; Honegr, J.; Musilek, K.; Kuca, K.* Ligand-based 3D QSAR analysis of reactivation potency of mono- and bis-pyridinium aldoximes toward VX-inhibited rat acetylcholinesterase. Journal of Molecular Graphics & Modelling. 2015, vol. 56, no. 1, p. 113-129. DOI: 10.1016/j.jmgm.2014.11.010
• Sharma, R.; Gupta, B.; Singh, N.; Acharya, J.R.; Musilek, K.; Kuca, K.; Ghosh, K.K.* Development and Structural Modifications of Cholinesterase Reactivators against Chemical Warfare Agents in Last Decade: A Review. Mini-Reviews in Medicinal Chemistry. 2015, vol. 15, no. 1, pp. 58-72. DOI: 10.2174/1389557514666141128102837
Surface modification of nanoparticles and cancer targets
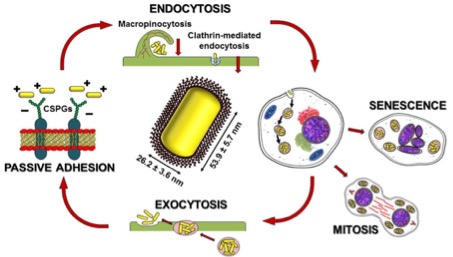
Cancer treatment is a worldwide issue, a lot of strategies are intensively researched and applied within its framework. Our team is trying to combat cancer through applying the theranostics strategy using gold nanorods. Up to now, investigations have been made into some surfactants valuable for modification of gold nanorods including their bioavailability or biocompatibility. The research team has developed and used a plenty of variable chemical agents valuable for this purpose. The focus is placed on the mechanism of the action plus safety of the used nanoparticles and their modifiers. The research is carried out in cooperation with our international partners.
Projects
• 18-14259S – Inhibition of JAK/STAT3 signalling pathway in cancer therapy
• 17-19968S – Localized Electronic Effects of Antibody Binding on NanoComposite Materials (LEEFAB)
Publications
• Zarska, M.; Novotny, F.; Havel, F.; Sramek, M.; Babelova, A.; Benada, O.; Novotny, M.; Saran, H.; Kuca, K.; Musilek, K.; Hvezdova, Z.; Dzijak, R.; Vancurova, M.; Krejcikova, K.; Gabajova, B.; Hanzlikova, H.; Kyjacova, L.; Bartek, J.; Proska, J.*; Hodny, Z.* A two-step mechanism of cellular uptake of cationic gold nanoparticles modified by (16-mercaptohexadecyl)trimethylammonium bromide (MTAB). Bioconjugate Chemistry. 2016, vol. 27, no. 10, p. 2558−2574. DOI: 10.1021/acs.bioconjchem.6b00491
• Zarska, M.; Sramek, M.; Novotny, F.; Havel, F.; Babelova, A; Mrazkova, B.; Benada, O.; Reinis, M.; Stepanek, I.; Musilek, K.; Bartek, J.; Ursinyova, M.; Novak, O.; Dzijak, R.; Kuca, K.*; Proska, J.*; Hodny, Z.* Biological safety and tissue distribution of (16-mercaptohexadecyl)trimethylammonium bromide-modified cationic gold nanorods. Biomaterials 2017, vol. 154, no. 1, p. 275-290. DOI: 10.1016/j.biomaterials.2017.10.044
Project supervisor
Section navigation: Topics

